In the United States, the Food and Drug Administration’s (FDA) Center for Devices and Radiological Health (CDRH) is responsible for regulating firms who manufacture, repackage, relabel, and/or import medical devices sold in the country.
When medical devices are registered with the FDA, they are classified into one of three general product classes: Class I, II, or III. While manufacturers must comply with basic regulatory requirements, such as establishment of registration, quality system regulation, and labeling requirements, regardless of device class, additional controls and requirements are based on the device’s general class and increase from Class I to Class III. Devices are sorted into their classification tier based on the product’s specifications, intended use (general purpose or function of the medical device), indications for use (the condition the medical device would be intended to diagnose, treat, prevent, or cure), and potential risk. Each classification has its own set of regulatory controls and requirements to ensure specific safety and efficacy standards are met, with requirements increasing for each tier.
Class I Devices:
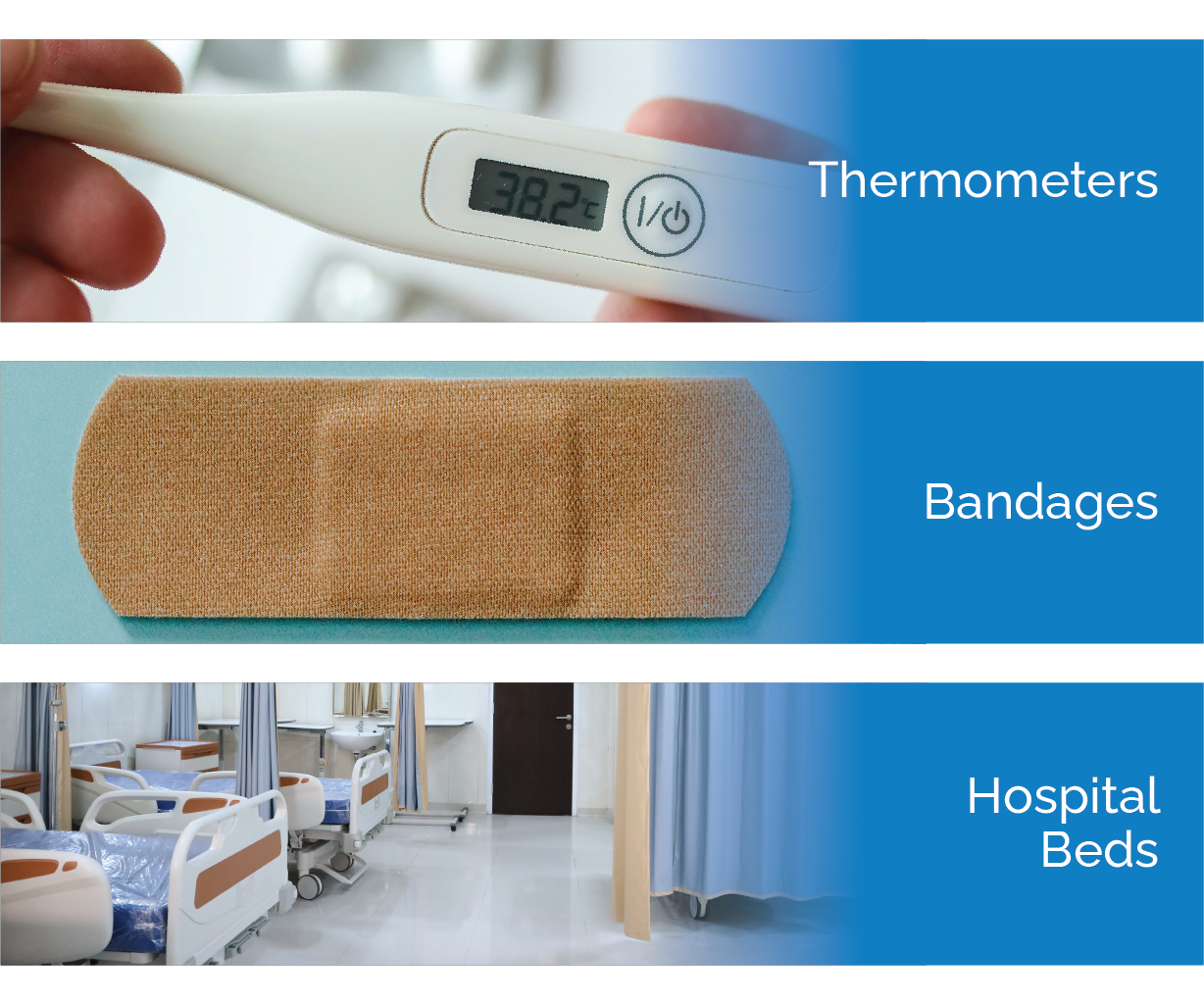
FDA defines class I devices as devices that are “not intended for use in supporting or sustaining life or of substantial importance in preventing impairment to human health, and they may not present a potential unreasonable risk of illness or injury.” Class I devices pose the lowest risk to users of the three tiers. Of all medical devices currently on the market, approximately 50% fall into this category. Many devices in this tier are exempt from 510(K) premarket notification, however, they are still subject to FDA general regulatory controls including provisions on misbranding, device registration, record keeping, and good manufacturing practices. Manufacturers of these items are required to implement quality management systems and uphold quality standards for medical products. Examples of medical devices in this tier are wheelchairs, bandages, hospital beds, and thermometers.
Examples of Class I Medical Devices
Class II Devices:
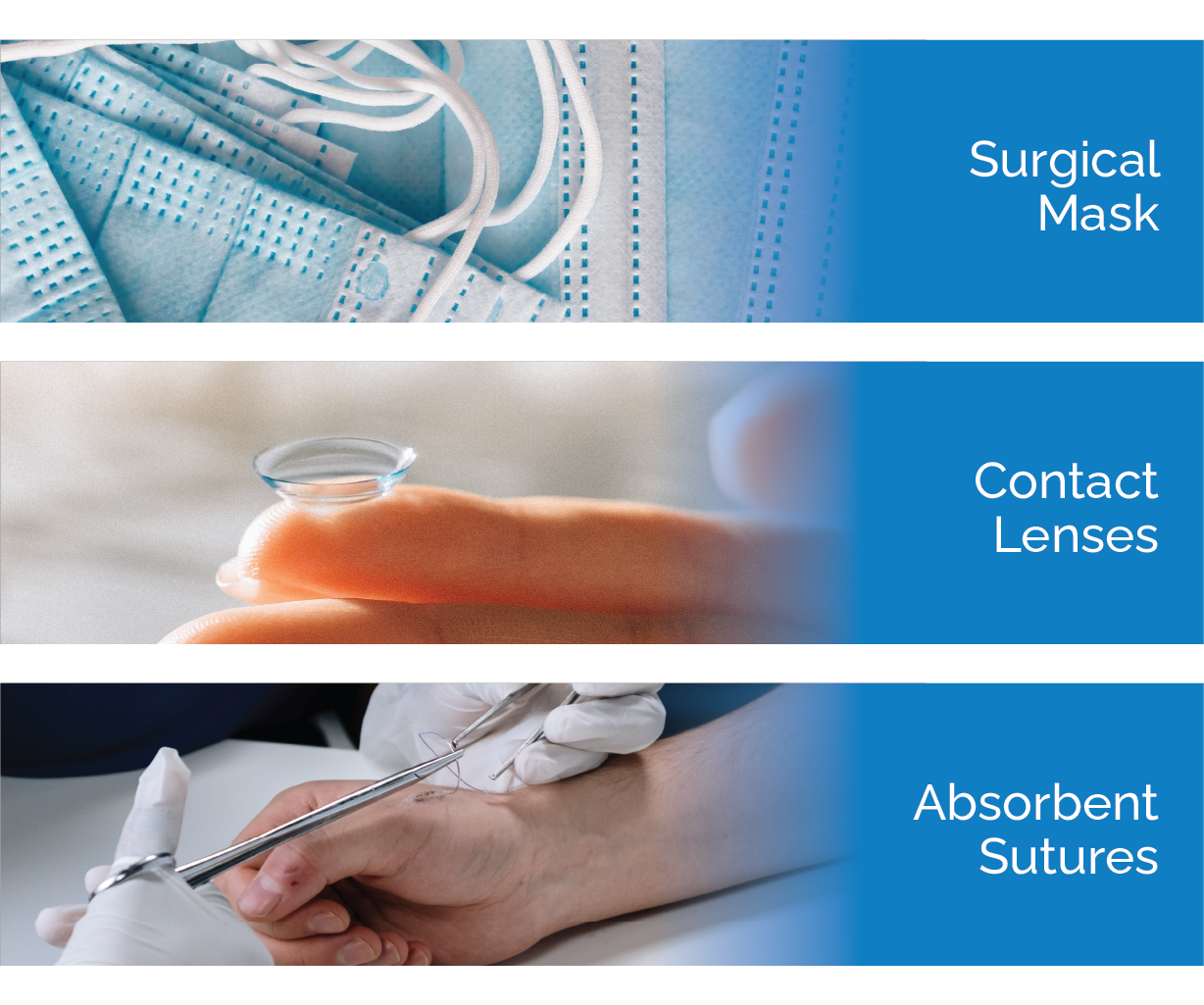
Class II devices pose a higher level of risk to patients or users as they are more likely to be in contact with the user and/or patient for a sustained period of time. There is also a higher potential for these devices to come into contact with the user’s cardiovascular system or internal organs. FDA defines these devices as devices where “general controls are insufficient to provide reasonable assurance of the safety and effectiveness of the device.” Of all medical devices currently on the market, approximately 40% fall into the class II tier. Devices in this tier are required to meet both general and specialized device-specific regulatory controls and submit for 510(K) premarket notification to gain FDA clearance prior to product marketing and distribution in the US. Examples of medical devices in this tier are catheters, contact lenses, absorbent sutures, and surgical masks.
Examples of Class II Medical Devices
Class III Medical Devices:
Class III Medical devices pose a high risk to patients and/or users and undergo the highest level of FDA scrutiny. FDA defines Class III devices as devices that “usually sustain or support life, are implanted, or present a potential unreasonable risk of illness or injury.” Only 10% of all medical devices on the market currently fit into this tier. Devices in this tier are required to receive Premarket Approval (PMA) from the FDA prior to marketing or distribution. The PMTA process is the most intensive type of device marketing application required by the FDA. As part of their PMA, these medical devices are required to undergo stringent testing and provide extensive clinical data to prove their safety and efficacy. Devices are classified to this tier for three reasons: the device is used in supporting or sustaining human life, of substantial importance in preventing impairment of human health, or presents a potential serious risk of illness or injury. Examples of these devices are cochlear implants, pacemakers, and wearable external defibrillators.
Examples of Class II Medical Devices




Leave A Comment