SOURCE: FDA CENTER FOR TOBACCO PRODUCTS
In order to provide the most current information, FDA will be regularly updating this page with reporting and progress of FDA intermediate and final actions taken on premarket applications– PMTA, SE Report and EX REQ – across the application review process. The information included in these PDF charts replaces the information previously posted on the Tobacco Product Marketing Orders page (which has now been updated to more clearly present the order letters, decision summaries and other documents associated with tobacco products that FDA has authorized). Those who were familiar with the previous monthly aggregate numbers posted on that page will note that the new PDF charts include data on many more categories and milestones in the review process.
FDA intends to provide these metrics and data on a regular and reliable basis and in an easy-to-understand format, typically within a month of the closing of the reporting period.
Note: Information on this page is current as of June 1, 2021. Due to the large volume of applications received in September in advance of the September 9 premarket application deadline, the exact total number of products within the applications is still being processed. This information will be updated in the tables when available.
FDA may periodically reassess and change the categories or amount of data provided on this website. This data is produced on an ongoing basis and is subject to change due to updates, corrections, or other reasons. Some metrics can also change as FDA is processing an extremely large number of applications that move through many steps during the review process. The data reported here is generally accurate to within 10%.
Most of the metrics reported reflect the number of tobacco products that are at each stage. Descriptions of each of the metrics are provided within the Tobacco Product Applications Metrics Glossary.
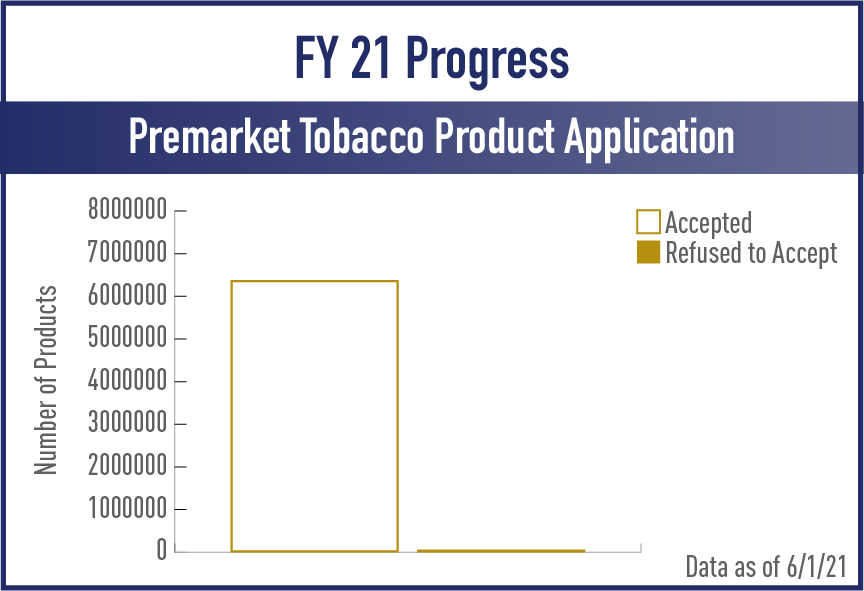
Premarket Tobacco Product Applications (PMTA)
FY2021
View the complete set of PMTA Metrics for Acceptance, Filing and Review/Action Phases
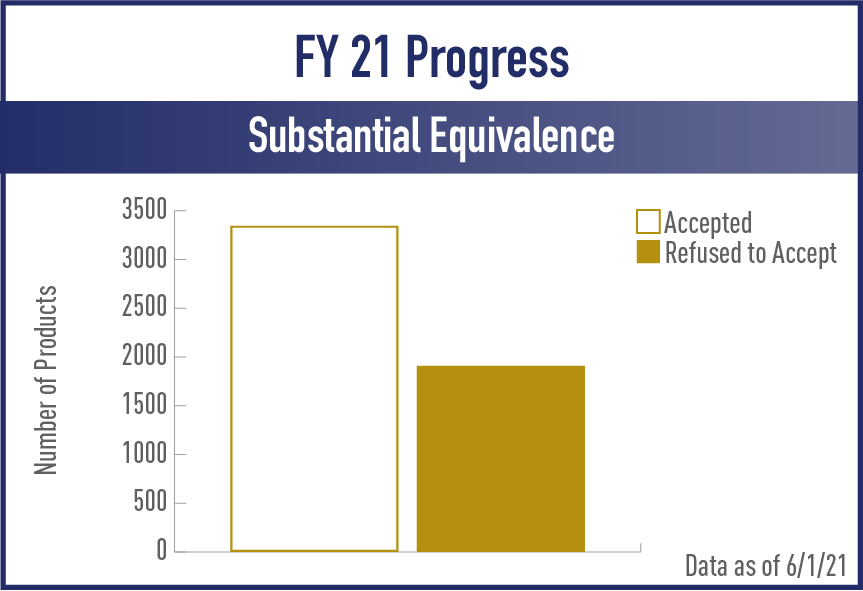
Substantive Equivalence (SE) Reports
FY2021
View the complete set of SE Report Metrics for Acceptance and Review/Action Phases
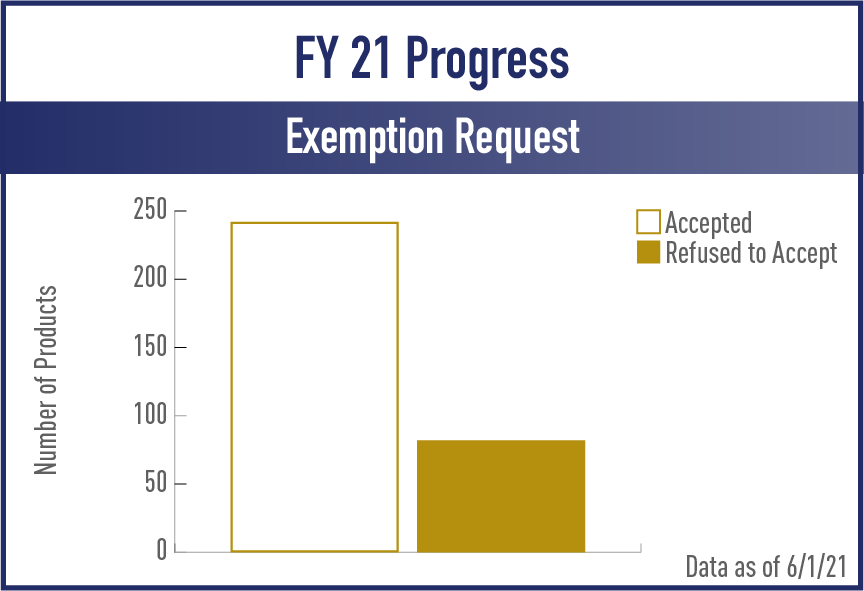
Exemption from Substantial Equivalence Requests (EXREQ)
FY2021
View the complete set of EX REQ Metrics for Acceptance and Review/Action Phases
Archive
Actions on PMTA, SE Report and EX REQs taken by FDA before FY 2021 can be found in the Archive.
Additional Resources



